COVID-19 Sample Collection Kits Market Growth & Trends
The global COVID-19 sample collection kits market size is expected to account for USD 3.9 billion by 2027, expanding at a CAGR of 4.9% over the forecast period, according to a new report by Grand View Research, Inc. The incorporation of 3-D printing technology is expected to enhance the production processes of the kits. The advent of 3-D printed swabs addresses the emergency shortages faced during the testing of coronavirus infection, which is expected to drive the market. Recently, in April 2020, the University of South Florida Health along with Northwell Health and Formlabs generated 3-D printed nasal swabs for safe and effective COVID-19 testing in patients.
Regulatory bodies are encouraging the development of efficient tests to ensure accurate and reliable diagnosis. Recently, in April 2020, the U.S. Food and Drug Administration (FDA) authorized the first at-home COVID-19 test, Pixel by LabCorp allowing patients to collect samples at home. This diagnostic test makes use of a Q-tip cotton nasal swab. This approval has enhanced the availability of a convenient and reliable at-home sample collection alternative for patients.
Several funding programs are expected to drive the production of sampling kits globally. For instance, in May 2020, Apple awarded USD 10 million to COPAN Diagnostics, an Italian company engaged in the production of sample collection kits, from its Advanced Manufacturing Fund. This funding would allow COPAN to boost its production and supply of kits from several thousands, currently, to more than 1 million COVID-19 sample collection kits per week by early July 2020.
Download Free Sample Report @
COVID-19 Sample Collection Kits Market Report Highlights
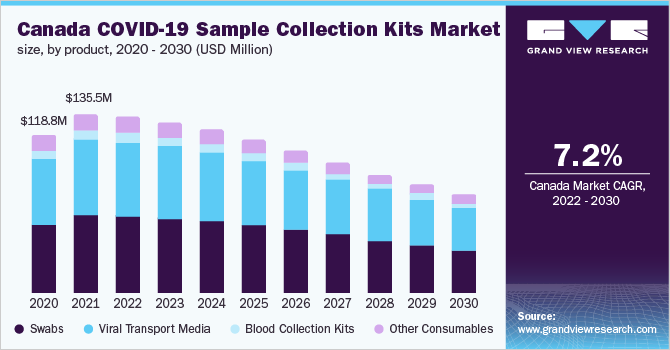
- Swabs segment is estimated to account for the largest revenue share with 43.0% as nasopharyngeal swabs are the preferred choice of specimen collection for upper respiratory tract infections as per recommendations of the World Health Organization (WHO)
- In addition, the large volume and usage rate of swabs can be attributed to the highest share of this product segment in the global market
- Diagnostics application segment accounts for the largest revenue share and is expected to expand at the fastest CAGR over the forecast period. This is attributed to the expansion of molecular diagnostic screening that has led to an increased usage of swabs and viral transport media for diagnostic applications
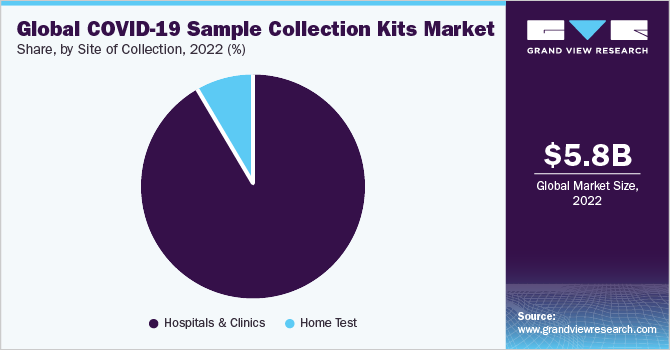
- A large volume of tests performed in hospitals and the efforts undertaken to improve the diagnostic methods for COVID-19 result in the dominant share of hospitals and clinics. For instance, Indian hospitals have designed airtight cabins for collecting swab samples for coronavirus testing to prevent the infection from spreading
- Europe accounted for the largest revenue share with the highest number of coronavirus cases globally. Domestic laboratories in Germany are conducting up to 160,000 tests per week. This rapid mass testing of coronavirus in Germany contributes to the region’s largest share
- Key market players, such as COPAN Diagnostics, Becton Dickinson, Puritan Medical Products, and Thermo Fisher, are constantly boosting the production of these kits
- As reported in March 2020, Puritan Medical Products was engaged in production of around 800,000 to 1 million swabs per week for testing coronavirus infection to overcome the shortages of swabs
The global COVID-19 sample collection kits market size was valued at USD 2.8 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 4.9% from 2020 to 2027. An exponential rise in the number of COVID-19 cases and inadequate laboratory-based molecular testing capacity are factors that have encouraged several companies to develop rapid and easy-to-use tests, which boosts the market growth. Furthermore, approval of synthetic swabs for use in the diagnosis of coronavirus infection and the subsequent increase in its production are expected to drive the market.
In April 2020, the Food and Drug Administration (FDA) approved the use of spun synthetic swabs that are similar to Q-tips swabs designed for COVID-19 testing. This approval allows patients to self-collect samples and minimize potential exposure to healthcare professionals. Furthermore, U.S. Cotton, one of the key manufacturers of cotton swabs in the U.S., is engaged in developing polyester swabs at a large scale to effectively address the growing need for COVID-19 diagnostic testing.
Have some specific queries about this report, our team of analyst will be glad to help!
The collection of samples is the most vital step in the laboratory diagnosis of several infectious diseases including coronavirus infection. Improper and inaccurate sample collection may lead to false or negative test results. Therefore, the Centers for Disease Control and Prevention (CDC) has recommended a standard sample collection procedure for specimen collection and transport with regard to SARS-CoV-2 testing. These regulatory guidelines have helped in streamlining the usage of COVID-19 sample collection kits.
Key participants are constantly accelerating the production and supply of specimen collection kits. The volume of swabs arriving from COPAN Diagnostics, Italy, in the U.S. is continuously increasing. Around 6 tons of swabs were imported in March 2020 and 4.5 tons of swabs in January 2020 from COPAN to the U.S. ports. On the other hand, Quest Diagnostics has distributed around 2 million swab collection kits in the U.S. as reported until May 2020. Moreover, a substantial number of companies from other domains are engaged in helping the government overcome the shortage of medical sterile swabs. As reported in May 2020, Royal DSM, a health, nutrition, and materials company, entered the swab manufacturing market to combat the shortage of specimen collection products in the Netherlands. This is the first time the company is generating swabs to meet the nation’s testing needs. The company is also donating 11 tons of material required for swab production in the country.
Some of the prominent players in the COVID-19 sample collection kits market include:
- Thermo Fisher Scientific, Inc.
- Puritan Medical Products
- COPAN Diagnostics
- Becton, Dickinson and Company
- Laboratory Corporation of America Holdings
- Lucence Diagnostics Pte Ltd.
- Hardy Diagnostics
- BNTX Inc.
- Formlabs
- Medline Industries, Inc.
- HiMedia Laboratories
- VIRCELL S.L.
As COVID-19 diagnostic testing continues to scale-up, several studies are being conducted to investigate different sample collection approaches that yield accurate results. A research study found that saliva samples collected from patients with the infection provide better detection sensitivity and consistency than the widely-used nasopharyngeal approach. It was also observed that there was low variability in results obtained using saliva samples, which is expected to expand their penetration of this approach in the future.
Check out special pricing optionsfor sectional purchase and startup companies
Grand View Research has segmented the global COVID-19 sample collection kits market on the basis of product, application, site of collection:
COVID-19 Sample Collection Kits Product Outlook (Revenue, USD Million, 2020 – 2027)
- Swabs
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Others
- Viral Transport Media
- Blood Collection Kits
- Other Consumables
COVID-19 Sample Collection Kits Application Outlook (Revenue, USD Million, 2020 – 2027)
- Diagnostics
- Research
COVID-19 Sample Collection Kits Site of Collection Outlook (Revenue, USD Million, 2020 – 2027)
- Hospitals & Clinics
- Home Test
About Grand View Research
Grand View Research, Inc. is a U.S. based market research and consulting company, registered in the State of California and headquartered in San Francisco. The company provides syndicated research reports, customized research reports, and consulting services. To help clients make informed business decisions, we offer market intelligence studies ensuring relevant and fact-based research across a range of industries, from technology to chemicals, materials and healthcare.
Media Contact
Company Name: Grand View Research, Inc.
Contact Person: Sherry James, Corporate Sales Specialist – U.S.A.
Email: Send Email
Phone: 1-415-349-0058, Toll Free: 1-888-202-9519
Address:201, Spear Street, 1100
City: San Francisco
State: California
Country: United States
Website: https://www.grandviewresearch.com/industry-analysis/covid-19-sample-collection-kits-market

