DelveInsight’s “Alopecia Areata Pipeline Insight” report provides comprehensive insights about 15+ companies and 15+ pipeline drugs in the Alopecia Areata pipeline landscapes. It comprises Alopecia Areata pipeline drug profiles, including clinical and non-clinical stage products. It also includes the Alopecia Areata therapeutics assessment by product type, stage, route of administration, and molecule type and further highlights the inactive Alopecia Areata pipeline products.
Some of the key takeaways of the Alopecia Areata Pipeline Report
- Concert Pharmaceuticals develops CTP-543 for the treatment of mild to severe forms of Alopecia Areata. The drug development company focuses majorly on developing novel drugs using its DCE Platform (deuterated chemical entity platform) to treat serious diseases and fulfil unmet patient needs.
- In November 2020, Concert Pharmaceuticals planned to initiate a multi-centre, double-blind, randomised, placebo-Controlled, Phase III study of CTP-543 for patients suffering from moderate to severe Alopecia Areata. The study is estimated to be completed by March 2022.
- On 8 July 2020, the U.S Food and Drug Administration (FDA) rewarded Breakthrough Therapy Designation to Concert Pharmaceutical’s oral Janus Kinase inhibitor, CTP-543, for the treatment of moderate to severe Alopecia Areata patients.
- CTP-543 has also received Fast Track designation for the Alopecia Areata treatment. The Breakthrough Therapy designation was awarded after analysing the Phase II trial data that demonstrated hair regrowth in patients suffering from this immune-mediated disorder.
- Many key players such as Arcutis Biotherapeutics, Bioniz Therapeutics, Pfizer, Eli Lilly and Company, Reistone Biopharma Company, Concert Pharmaceuticals, and others involved in targeted Alopecia Areata therapeutics development and fulfil unmet patient needs.
- On March 3, 2021, Eli Lilly and Company and Incyte announced today top-line results from BRAVE-AA2, a Phase 3 study evaluating the efficacy and safety of once-daily baricitinib 2-mg and 4-mg in adults with severe alopecia areata (AA). Baricitinib is First JAK-Inhibitor to Demonstrate Hair Regrowth in Phase 3 Alopecia Areata (AA) Trial.
- Arena Pharmaceuticals is running a phase 2 clinical trial where they’re studying a sphingosine-1-modulator known as etrasimod. Pfizer is conduction a phase 3 clinical trial of PF-06651600 (Emt protein-tyrosine kinase inhibitors; Janus kinase 3 inhibitors) for the Treatment of Alopecia Areata.
Get an overview of pipeline landscape @ Alopecia Areata Clinical Trial Analysis
Alopecia Areata is an autoimmune skin disorder in which the hair follicles get damaged by a faulty immune system. This faultiness causes the immune system to attack its own body, disrupting hair follicles and new hair formation. The disorder can be equally witnessed in males and females, although the occurrence is mainly seen in people below 30 years. Alopecia Areata treatments include Injections, Oral, Topical Treatments and Light therapy.
Alopecia Areata Pipeline Therapeutics
- CTP-543: Concert Pharmaceuticals
Concert Pharmaceuticals is developing CTP-543 to treat mild to severe forms of Alopecia Areata. The drug acts as an oral inhibitor of Janus Kinases JAK1 AND JAK2. The research studies performed have evidently shown that inhibition of the JAKs enzymes can prove to be beneficial in the treatment of autoimmune disorders. The drug molecule CTP-543 is a deuterium modified version of the JAK1/2 inhibitor Ruxolitinib.
Research and Development
Phase III
NCT04518995: In November 2020, Concert Pharmaceuticals planned to initiate a multi-centre, double-blind, randomised, placebo-controlled, Phase III study of CTP-543 for patients suffering from moderate to severe Alopecia Areata. The study is estimated to be completed by March 2022, with an estimated enrollment of 700 participants.
For further information, refer to the detailed report @ Alopecia Areata Emerging Drugs
Scope of Alopecia Areata Pipeline Drug Insight
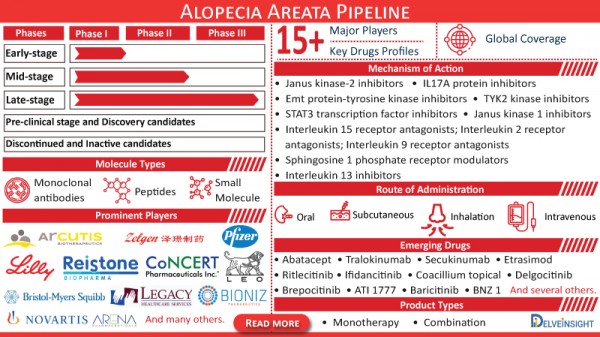
- Coverage: Global
- Major Players:15+ Key Players
- Prominent Players: Arcutis Biotherapeutics, Bioniz Therapeutics, Pfizer, Eli Lilly and Company, Reistone Biopharma Company, Concert Pharmaceuticals, Suzhou Zelgen Biopharmaceuticals, Legacy Healthcare, Arena Pharmaceuticals, LEO Pharma, Aclaris Therapeutics, Bristol-Myers Squibb, Novartis, and many others.
- Key Drugs Profiles: 15+ Products
- Key Products: Abatacept, Tralokinumab, Secukinumab, BNZ 1, Ritlecitinib, Ifidancitinib, Coacillium topical, Delgocitinib, Etrasimod, Brepocitinib, ATI 1777, Baricitinib, and several others.
- Phases:
- Alopecia Areata Therapies Late-stage (Phase III)
- Alopecia Areata Therapies Mid-stage (Phase II)
- Alopecia Areata Therapies Early-stage (Phase I)
- Alopecia Areata Pre-clinical stage and Discovery candidates
- Discontinued and Inactive candidates
- Molecule Types:
- Small Molecule
- Monoclonal antibodies
- Peptides
- Mechanism of Action:
- Janus kinase-2 inhibitors
- Emt protein-tyrosine kinase inhibitors
- STAT3 transcription factor inhibitors
- TYK2 kinase inhibitors
- Janus kinase 1 inhibitors
- Interleukin 15 receptor antagonists; Interleukin 2 receptor antagonists; Interleukin 9 receptor antagonists
- Interleukin 13 inhibitors
- IL17A protein inhibitors
- Sphingosine 1 phosphate receptor modulators
- Route of Administration:
- Inhalation
- Intravenous
- Oral
- Subcutaneous
- Product Types:
- Monotherapy
- Combination
Key Questions regarding Current Alopecia Areata Treatment Landscape and Emerging Therapies Answered in the
Pipeline Report
- What are the current options for Alopecia Areata treatment?
- How many companies are developing therapies for the treatment of Alopecia Areata?
- How many are Alopecia Areata emerging therapies in the early-stage, mid-stage, and late stages of development to treat Alopecia Areata?
- What are the key collaborations (Industry-Industry, Industry-Academia), Mergers and acquisitions, and significant licensing activities that will impact the Alopecia Areata market?
- Which are the dormant and discontinued products and the reasons for the same?
- What is the unmet need for current therapies for the treatment of Alopecia Areata?
- What are the current novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing Alopecia Areata therapies?
- What are the critical designations that have been granted for the emerging therapies for Alopecia Areata?
- How many patents are granted and pending for the emerging therapies to treat Alopecia Areata?
Table of Contents
1. Alopecia Areata Report Introduction
2. Alopecia Areata Executive Summary
3. Alopecia Areata Overview
4. Alopecia Areata Analytical Perspective In-depth Commercial Assessment
5. Alopecia Areata Pipeline Therapeutics
6. Alopecia Areata Late-Stage Products (Phase III)
6.1. CTP-543: Concert Pharmaceutical
7. Alopecia Areata Mid-Stage Products (Phase II)
7.1. SHR0302: Reistone Biopharma Company
8. Alopecia Areata Early-Stage Products (Phase I)
8.1. BNZ-1: Bioniz Therapeutics
9. Alopecia Areata Preclinical Stage Products
9.1. ARQ 255: Arcutis Biotherapeutics
10. Alopecia Areata Therapeutic Assessment
11. Alopecia Areata Inactive Products
12. Company-University Collaborations (Licensing/Partnering) Alopecia Areata Analysis
13. Alopecia Areata Key Companies
14. Alopecia Areata Key Products
15. Alopecia Areata Unmet Needs
16 . Alopecia Areata Market Drivers and Barriers
17. Alopecia Areata Future Perspectives and Conclusion
18. Alopecia Areata Analyst Views
19. Appendix
20. About DelveInsight
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email: Send Email
Phone: +19193216187
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/report-store/alopecia-areata-pipeline-insight?utm_source=SatPR&utm_medium=pressrelease&utm_campaign=apr

