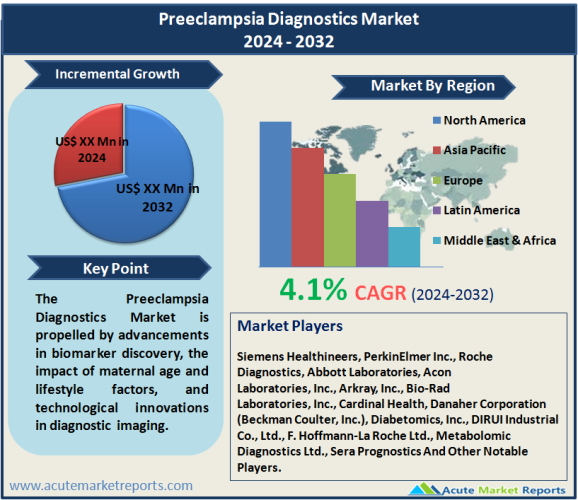
The preeclampsia diagnostics market is expected to grow at a CAGR of 4.1% during the forecast period of 2024 to 2032. This growth will be driven by developments in the discovery of biomarkers, the influence of maternal age and lifestyle factors, and technological advances in diagnostic imaging. Nevertheless, the lack of knowledge and accessibility to diagnostic tools in resource-constrained environments presents a significant obstacle, underscoring the need for fair healthcare approaches to tackle the worldwide issue of preeclampsia. Intriguing insights are revealed by the market segmentation, with distinct segments exhibiting the highest CAGR and revenue leadership. In contrast to the revenue dominance of blood tests, urine tests demonstrate the most substantial compound annual growth rate (CAGR), which underscores the sector’s emphasis on economical and non-invasive diagnostic alternatives. The revenue of instruments is the highest among products and services, while the highest CAGR is observed in the services sector, underscoring the significance of comprehensive diagnostic solutions. The revenue generated by hospitals is the highest, underscoring their pivotal position, whereas the CAGR of diagnostic centers indicates a transition towards specialized facilities. From a geographic standpoint, North America generates the largest revenue share due to its firmly established healthcare infrastructure. Conversely, the Asia-Pacific region demonstrates the highest CAGR, which is indicative of swift economic progress and heightened healthcare spending. Europe sustains a consistent level of contribution, whereas the Middle East Africa, and Latin America exhibit prospects for expansion. Siemens Healthineers, PerkinElmer Inc., and Roche Diagnostics emerge as significant contenders in the competitive environment, employing tactics including product diversification, strategic collaborations, and research and development. The competitive dynamics of the market are influenced by entities that demonstrate a dedication to innovation, customer-centric strategies, and market differentiation via specialized diagnostic solutions.
Key Market Drivers
The persistent endeavor to identify precise and timely preeclampsia is notably accelerated by developments in biomarker identification. An ongoing effort is being made by researchers and diagnostic companies to identify and validate new biomarkers that are linked to preeclampsia, with the ultimate goal of improving the accuracy of diagnostics. Significantly, attention has been drawn to the identification of placental growth factor (PlGF) as a biomarker. When combined with other biomarkers such as pregnancy-associated plasma protein-A (PAPP-A) and soluble fms-like tyrosine kinase-1 (sFlt-1), PlGF aids in the advancement of complex diagnostic assays. The clinical studies provide substantial evidence supporting the effectiveness of these biomarkers. Their diagnostic potential has been validated through the publication of research in prestigious journals, including “The New England Journal of Medicine” and “Hypertension in Pregnancy.”
Significantly contributing to the increased incidence of preeclampsia is the increasing prevalence of lifestyle factors and the upward trend in maternal age; consequently, this increases the demand for sophisticated diagnostics. Pregnancies that occur later in life, which are frequently linked to older mothers, increase the likelihood of developing preeclampsia. Additionally, sedentary behavior and obesity are lifestyle factors that serve to amplify this risk. Several research studies, such as those that have been published in “PLOS ONE” and “American Journal of Obstetrics and Gynaecology,” provide further insight into the relationship that exists between preeclampsia risk and maternal age and lifestyle. Due to the potential complications associated with preeclampsia, the urgency to identify the condition early motivates the implementation of diagnostic instruments in clinical environments.
Significant Market Drivers for preeclampsia diagnostics Technological advancements in diagnostic imaging, specifically in the domains of ultrasound and Doppler velocimetry, are technological innovations in diagnostic imaging. Sophisticated imaging modalities enable the implicit evaluation of fetal and maternal blood circulation, thereby assisting medical professionals in the timely identification and surveillance of preeclampsia. Doppler ultrasound, specifically, enables the evaluation of blood flow through the uterus and umbilical arteries, thereby offering significant insights into alterations in vascular resistance and hemodynamics that may be linked to preeclampsia. The diagnostic precision and clinical management guidance provided by these imaging modalities are supported by peer-reviewed articles published in “Ultrasound in Obstetrics & Gynaecology” and “American Journal of Perinatology.”
Browse for report at : https://www.acutemarketreports.com/report/preeclampsia-diagnostics-market
Market Restraint: In settings with limited resources, awareness and access to diagnostics are restricted.
One notable obstacle impeding the extensive implementation of preeclampsia diagnostics is the restricted knowledge and availability of sophisticated diagnostic instruments, especially in settings with limited resources. Although the variables mentioned above primarily emphasize technological progress and the identification of biomarkers, resource-constrained regions present practical obstacles. The evidence presented in studies published in reputable journals such as “Global Health: Science and Practice” and “The Lancet” underscores the inequities in diagnostic technology accessibility that result in delayed or overlooked diagnoses among specific populations. The insufficient knowledge and understanding regarding the critical nature of early preeclampsia detection among healthcare professionals and expectant mothers exacerbate this inhibition, underscoring the immediate necessity for focused interventions and awareness initiatives.
Market by Test Type
The test type category is a critical factor in determining the industry landscape of the preeclampsia diagnostics market, which is segmented according to test type. The Blood Test segment establishes itself as the leading contributor, attaining the greatest revenue in 2023. The prevailing status of blood-based biomarkers, such as Placental Growth Factor (PlGF), Soluble Fms-like Tyrosine Kinase-1 (sFlt-1), and Pregnancy-Associated Plasma Protein-A (PAPP-A), can be ascribed to their firmly established effectiveness. By their extensive research and validation in studies published in reputable journals, these biomarkers enhance the precision and dependability of blood tests utilized for the diagnosis of preeclampsia. On the contrary, the Urine Test segment emerges as the focal point due to its highest CAGR projected for the period spanning from 2024 to 2032. Sustained investigation into urine-based biomarkers and the growing acceptance of urine tests position this market segment for substantial expansion. Urine tests’ projected high CAGR is underscored by the fact that their non-invasive and cost-effective characteristics are in line with evolving diagnostic preferences.
Market by Offerings
The market is segmented according to product and service offerings, which offers valuable information regarding the revenue patterns and future development of the preeclampsia diagnostics industry. Instruments will emerge as the primary source of revenue in the year 2023. The demand for sophisticated imaging tools and diagnostic instruments that are essential for precise preeclampsia diagnosis is the driving force behind this prominence. Nevertheless, when the highest CAGR is accounted for from 2024 to 2032, the Services sector emerges as the focal point. The projected growth trajectory for services including diagnostic consultation, interpretation, and counseling is substantial. Complementing conventional product offerings, the emphasis on comprehensive diagnostic services is consistent with the evolution of healthcare delivery models and highlights the expected high CAGR.
Market by End Users
Commercial by End-Use Segmentation based on end-use provides insight into the significant influence that various healthcare settings have on the implementation of preeclampsia diagnostics. As 2023 progresses, hospitals assume a pivotal role in generating revenue, underscoring their essential function in providing antenatal care and managing high-risk pregnancies. On the contrary, the Diagnostic Centres sector exhibits the most substantial CAGR from 2024 to 2032. This highlights a significant transition towards specialized facilities that are solely focused on sophisticated and streamlined preeclampsia diagnostics. The rising demand for specialized diagnostic services outside of conventional hospital environments corresponds to the anticipated substantial CAGR for diagnostic centers. This signifies a substantial paradigm shift in the provision of healthcare services for the diagnosis of preeclampsia.
North America Continues to be the Global Leader
North America is a significant contributor, accounting for the maximum proportion of revenue in 2023. The aforementioned supremacy can be ascribed to the presence of sophisticated diagnostic technologies, a strong emphasis on research and development, and a firmly established healthcare infrastructure. Due to the region’s proactive stance and high levels of awareness regarding maternal healthcare, it holds a significant market share. On the contrary, it is observed that the Asia-Pacific region experiences the most substantial CAGR from 2024 to 2032, as projected. Significant growth is observed in this region as a result of a burgeoning emphasis on maternal and fetal health, accelerated economic development, and rising healthcare expenditures. Population demographics, urbanization, and increased awareness all contribute to a surge in demand for preeclampsia diagnostics, especially in countries such as China and India. Europe consistently maintains a significant share of the market’s revenue, owing to its aging population and firmly established healthcare systems. Latin America and the Middle East & Africa exhibit significant growth potential, despite commencing with a relatively lower revenue percentage. Government initiatives, increasing public consciousness, and the enhancement of healthcare infrastructure all contribute to the upward trend in these regions. The aforementioned geographic patterns represent an intricate relationship among regional healthcare dynamics, economic progress, and levels of awareness, all of which collectively impact the worldwide trajectory of the preeclampsia diagnostics market.
Focus on R&D to Increase Market Share Among Leading Rivals
Prominent organizations including Siemens Healthineers, PerkinElmer Inc., Roche Diagnostics, Abbott Laboratories, Acon Laboratories, Inc., Arkray, Inc., Bio-Rad Laboratories, Inc., Cardinal Health, Danaher Corporation (Beckman Coulter, Inc.), Diabetomics, Inc., DIRUI Industrial Co., Ltd., F. Hoffmann-La Roche Ltd., Metabolomic Diagnostics Ltd., and Sera Prognostics demonstrate a steadfast dedication to research, strategic partnerships, and innovation. Siemens Healthineers, a market leader, is committed to producing point-of-care testing and sophisticated diagnostic solutions, such as immunoassays, to improve the precision and efficacy of preeclampsia diagnostics. Constricted to the discovery of biomarkers for preeclampsia, PerkinElmer Inc. places a strong emphasis on research and development to broaden its portfolio of diagnostic instruments. The company is renowned for its comprehensive healthcare solutions. Roche Diagnostics, a prominent multinational corporation, utilizes its vast network and technological prowess strategically to offer comprehensive diagnostic solutions that aid in the timely identification and treatment of preeclampsia. A primary approach adopted in the preeclampsia diagnostics industry is to prioritize research and development to facilitate the advancement of diagnostic technologies and the identification of biomarkers. Prominent entities dedicate substantial resources to maintaining a leading position in innovation, consistently enhancing the precision and effectiveness of preeclampsia diagnostic instruments. Strategic collaborations and partnerships are of utmost importance, as organizations establish alliances with healthcare providers, research institutions, and other participants in the industry. The purpose of these collaborative efforts is to exchange knowledge, gain access to a variety of datasets, and resolve the difficulties associated with preeclampsia diagnosis as a group. In addition, a noteworthy trend observed among leading companies is the expansion of their product portfolios through diversification. Prominent healthcare providers and expectant women are catered to by the extensive array of diagnostic tools, instruments, and services provided by Siemens Healthineers, PerkinElmer Inc., and Roche Diagnostics.
Media Contact
Company Name: Acute Market Reports, Inc.
Contact Person: Chris Paul
Email: Send Email
Phone: US/Canada: +1-855-455-8662, India: +91 7755981103
Address:90 Church St, FL 1 #3514, New York, NY 10008, USA
City: New York
State: New York
Country: United States
Website: www.acutemarketreports.com