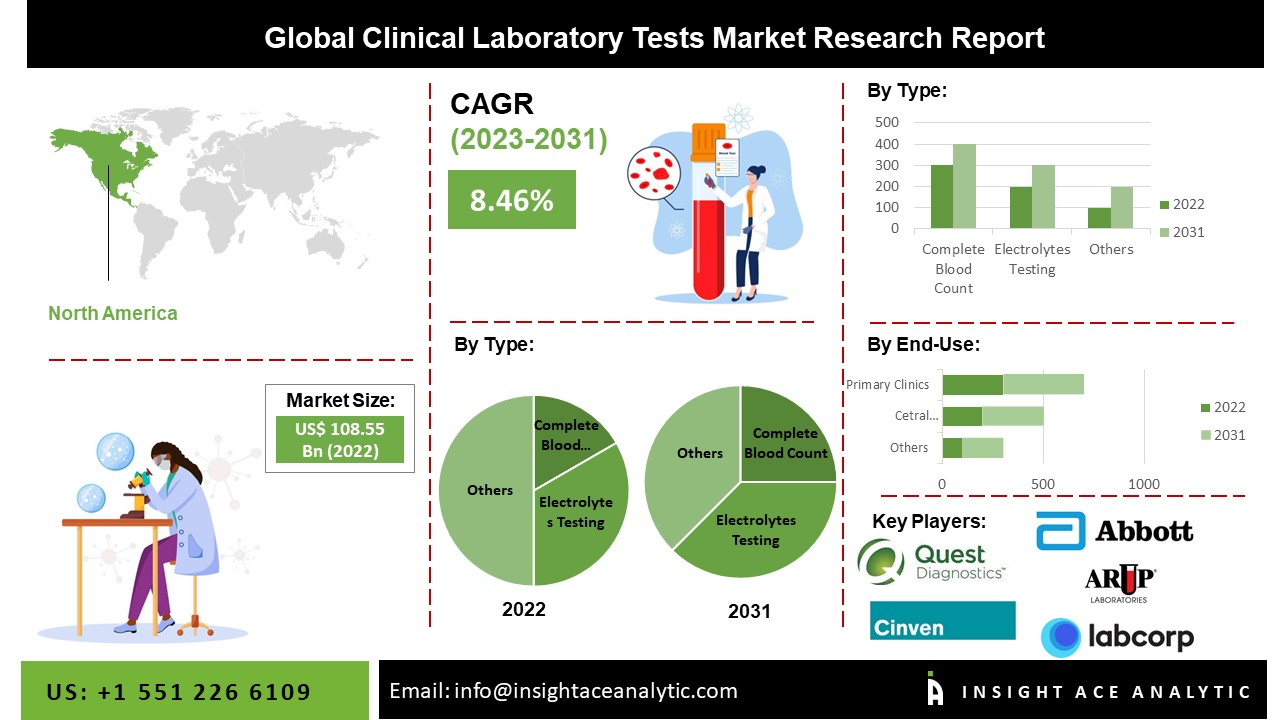
GlobalClinical Laboratory Tests Marketis valued at US$ 116.06 Bn in 2023, and it is expected to reach US$ 224.53 Bn by 2031, with a CAGR of 8.74% during the forecast period of 2024-2031.
Clinical laboratory tests are diagnostic medical procedures that examine samples from the body, such as blood, urine, or tissue, in order to identify health issues, inform treatment choices, and track the development of diseases. These tests are crucial in the field of healthcare as they serve the purpose of identifying illnesses, monitoring chronic conditions, evaluating the efficacy of therapies, and performing health screenings. The clinical laboratory tests market is expanding due to reasons such as an ageing population, an increase in the prevalence of target diseases, and the emergence of novel solutions to meet the market’s growing need for clinical lab testing. The rising prevalence of disorders like cardiovascular disease and diabetes is likely to be a major driver of market development over the forecast period.
Recent Developments:
- In September 2022, Bionano Genomics Inc. announced the formation of Bionano Laboratories, which will integrate Bionano’s optical genome mapping (OGM) data services with Lineagen’s clinical testing services. It also announced the introduction of Bionano Laboratories’ first OGM-based laboratory-developed test (LDT).
- In Feb 2022, Mindray introduced its latest BC-700 Series haematology analyzers for small- to medium-sized labs, which include both complete blood count (CBC) and erythrocyte sedimentation rate (ESR) assays.
Download Free Report Sample Pages:https://www.insightaceanalytic.com/request-sample/1689
Market Dynamics:
Market Drivers: Advancements in Technology
Continuous advancements in diagnostic technology, such as molecular diagnostics, next-generation sequencing, and automation, improve the accuracy, speed, and efficiency of laboratory testing. These advances broaden the spectrum of tests accessible and enhance diagnostic skills. The rise of personalized medicine, which tailors treatment strategies based on individual genetic makeup, biomarker profiles, and other patient-specific characteristics, has increased demand for molecular and genetic testing.
Challenges: High Cost of Developing and Validating New Laboratory Tests
The high expense of developing and validating new laboratory tests, particularly complicated molecular and genetic assays, may limit their availability and use, especially in resource-constrained contexts. Furthermore, pricing demands from payers and healthcare providers may lead to lower test prices, affecting laboratory income and profitability. Furthermore, the other factors impeding market expansion are stringent government guidelines and regulatory requirements for laboratory research. Clinical laboratory products are regulated by country-specific organizations to ensure their quality.
North America Is Estimated To Grow With The Maximum CAGR During The Forecast Period
The North American Clinical Laboratory Tests Market is likely to record a significant revenue share and to develop at a rapid CAGR in the near future. This can be ascribed to the growing elderly population, increased prevalence of chronic diseases such as cancer, and high market penetration of technologically sophisticated diagnostic techniques, which are likely to fuel market growth throughout the projection period. The regional market is estimated to be propelled by an increasing inclination for novel techniques as well as rising patient awareness.
Segmentation of Clinical Laboratory Tests Market-
Clinical Laboratory Tests Market By Product-
- Complete Blood Count
- HGB/ HCT testing
- Basic Metabolic Panel Testing
- BUN Creatinine Testing
- Electrolytes Testing
- HbA1c Testing
- Comprehensive Metabolic Panel Testing
- Liver Panel Testing
- Hepatitis
- Bile Duct Obstruction
- Liver Cirrhosis
- Liver Cancer
- Bone Disease
- Autoimmune Disorders
- Others
- Renal Panel Testing
- Lipid Panel Testing
- Cardiovascular Panel Tests
Clinical Laboratory Tests Market By End-Use
- Central Laboratories
- Complete Blood Count
- HGB/ HCT testing
- Basic Metabolic Panel Testing
- BUN Creatinine Testing
- Electrolytes Testing
- HbA1c Testing
- Comprehensive Metabolic Panel Testing
- Liver Panel Testing
- Renal Panel Testing
- Lipid Panel Testing
- Cardiovascular Panel Tests
- Primary Clinics
- Complete Blood Count
- HGB/ HCT testing
- Basic Metabolic Panel Testing
- BUN Creatinine Testing
- Electrolytes Testing
- HbA1c Testing
- Comprehensive Metabolic Panel Testing
- Liver Panel Testing
- Renal Panel Testing
- Lipid Panel Testing
- Cardiovascular Panel Tests
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- South East Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
Order the 180 Pages Detailed Report : https://www.insightaceanalytic.com/customisation/1689
Call: North America +1 551 226 6109 Email: diana.dsouza@insightaceanalytics.com
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/