InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global Celecoxib API Market- (By Type (Standard Grade Celecoxib API, & Micronized Celecoxib API), By Application (Pharmaceutical, & Laboratory)) Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
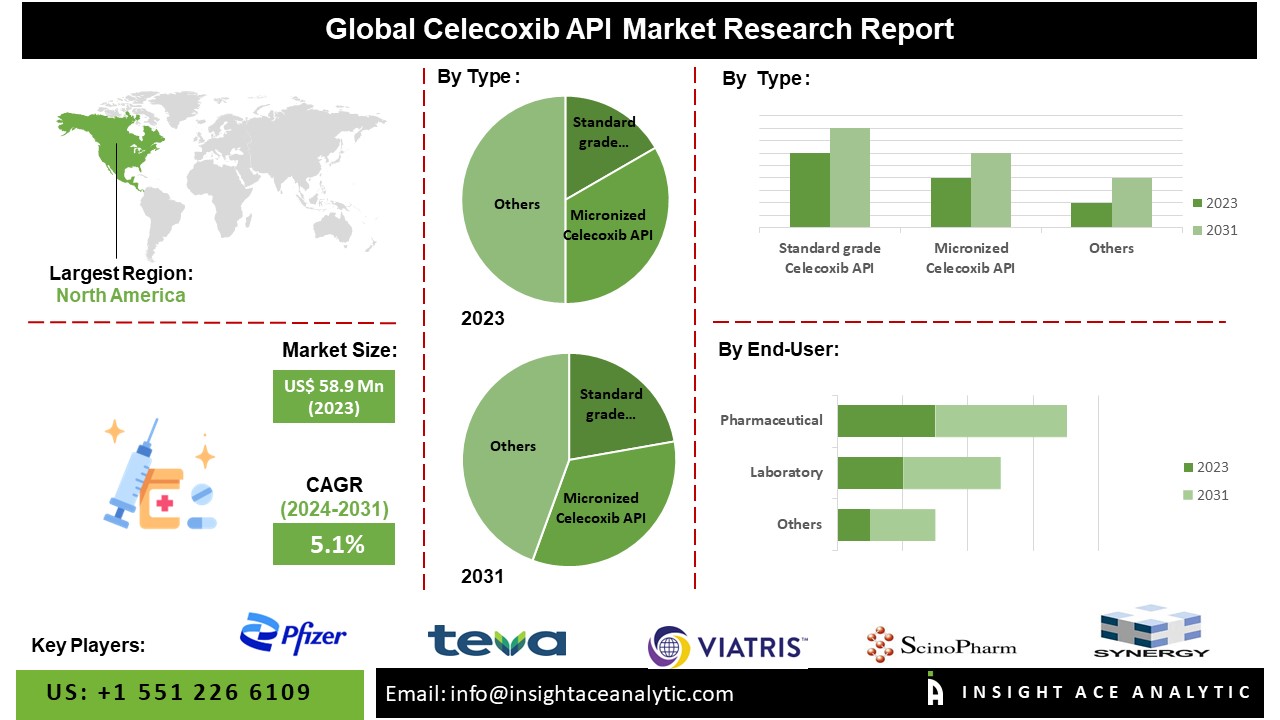
According to the latest research by InsightAce Analytic, the Global Celecoxib API Market is valued at US$ 58.9 Mn 2023, and it is expected to reach US$ 87.0 Mn by 2031, with a CAGR of 5.1% during the forecast period of 2024-2031.
Celecoxib API pertains to the active pharmaceutical ingredient of celecoxib, which is the essential element accountable for the medication’s medicinal effects. Specifically categorized as a selective COX-2 inhibitor, celecoxib is a nonsteroidal anti-inflammatory medication. The worldwide celecoxib API market is expanding robustly, propelled by many important factors. There is a growing need for effective and high-quality active pharmaceutical ingredients, such as celecoxib, to alleviate inflammation and discomfort. Celecoxib active pharmaceutical ingredients are in high demand because pharmaceutical companies are putting a lot of effort into creating effective treatments for illnesses like arthritis.
Moreover, a new development in the worldwide celecoxib API industry is the increasing emphasis on environmentally conscious and sustainable manufacturing methods. Industry participants are investigating more environmentally friendly methods to produce celecoxib as environmental consciousness grows in importance within the pharmaceutical manufacturing process. This will also expand the market growth in the coming years.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/2474
List of Prominent Players in the Celecoxib API Market:
- Pfizer
- Teva Pharmaceuticals
- Viatris
- ScinoPharm
- Jiangxi Synergy
- Jiangsu Hengrui Medicine
- Jiangsu Chiatai Qingjiang
- Hisun Pharmaceutical Nantong
- Aurobindo Pharma
- Punjab Chemicals
Market Dynamics:
Drivers-
The growing demand for the celecoxib API market is fueled by the fact that it is extensively used to control pain and inflammation, especially in inflammatory joint diseases, which is growing the market growth. Furthermore, the prevalence of these chronic illnesses is rising with the age of the world’s population, which is also driving market growth. Furthermore, generic manufacturers have entered the market due to the expiration of important patents, which has increased accessibility and decreased costs for patients. Market demand for celecoxib is expected to be further boosted by the ongoing research into its therapeutic potential in several areas, including cancer prevention and psychiatric diseases. Innovations in pharmaceutical technology that enhance celecoxib’s formulation also contribute to the industry’s worldwide market growth.
Challenges:
One of the main obstacles is the complex regulatory landscape that pharmaceutical manufacturers must navigate. This environment requires extensive testing, quality control, and documentation to ensure compliance, all of which drive up production costs and impede market expansion. The need for Celecoxib API may also decline if more effective and safer alternatives to the current pain and inflammation management options become available. Problems with Celecoxib’s solubility and stability, among other technical challenges in API synthesis, pose a threat to the market since they can make formulation processes more complicated and reduce the therapeutic value of drugs. As a whole, these factors slow down the growth of the market celecoxib API.
Regional Trends:
The North American celecoxib API market is anticipated to register a major market share in revenue. It is projected to grow at a high CAGR in the near future because of a prosperous pharmaceutical sector that boasts state-of-the-art research and production facilities. Celecoxib pharmaceutical products, including active pharmaceutical ingredients, are efficiently produced and distributed in the region due to their strong infrastructure. Besides, Europe has a substantial market share because celecoxib has a large customer base in the region because of the significant number of people living with chronic pain and inflammatory disorders. Europe’s enormous patient population fosters consistent expansion and importance, robust regulatory frameworks, and advantageous reimbursement policies.
Enquiry Before Buying: https://www.insightaceanalytic.com/enquiry-before-buying/2474
Recent Developments:
- In April 2024, Pfizer Inc. revealed excellent top-line immunogenicity and safety findings from the current pivotal Phase 3 clinical trial called MONeT (RSV IMmunizatiON Study for AdulTs at Higher Risk of Severe Illness). In people 18 to 59 years old who were at risk of developing severe respiratory syncytial virus-associated lower respiratory tract disease, a single dosage of ABRYSVO was compared with a placebo in this experiment.
- In January 2024, Teva announced that it would be selling its business related to active pharmaceutical ingredients (APIs). TAPI, a global leader in the small-molecule API market, employs about 4,300 people.
Segmentation of Celecoxib API Market-
By Type
- Standard Grade Celecoxib API
- Micronized Celecoxib API
By Application
- Pharmaceutical
- Laboratory
By Region
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
For More Customization: https://www.insightaceanalytic.com/customisation/2474
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/