Several major pharma and biotech giants are persistently working towards the development of novel treatment therapies that can address the defects caused by FGFR3 gene mutation. There are several promising drugs in the pipeline, including TransCon CNP (Ascendis Pharma), Infigratinib (BridgeBio), Recifercept (Pfizer), and RBM-007 (Ribomic Inc), among others.
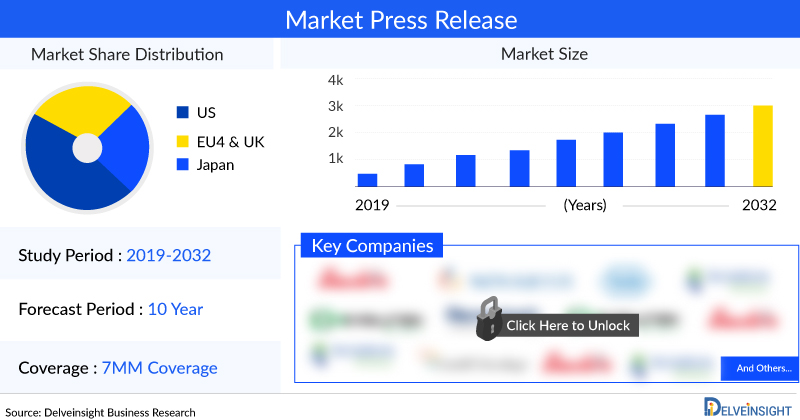
DelveInsight’s “Achondroplasia Market Insights, Epidemiology, and Market Forecast 2032” report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the Achondroplasia market size, share, trends, and growth opportunities in the seven major markets (7MM) (i.e., the United States, EU4 (Germany, Spain, Italy, France), the United Kingdom and Japan).
The Achondroplasia market report covers emerging drugs, current treatment practices, market share of individual therapies, and current & forecasted market size from 2019 to 2032. It also evaluates the current treatment practice/algorithm, key drivers & barriers impacting the market growth, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Achondroplasia Overview
Achondroplasia is a genetic disorder characterized by an unusually large head (macrocephaly), short upper arms (rhizomelic dwarfism), and short stature (adult height of approximately 4 feet). FGFR genetic alterations can cause more than 99% of Achondroplasia cases. In a small proportion of infants, symptoms like excessive accumulation of fluid around the brain (hydrocephalus) and low muscle tone (hypotonia) may happen as typical of achondroplasia. Acquisition of developmental motor milestones may also be delayed.
Achondroplasia results in short-limb skeletal dysplasia, occurring in approximately 1 in 20,000-30,000 live births. This genetic disorder is caused by a mutation in the fibroblast growth factor receptor 3 (FGFR3) gene, which occurs due to a spontaneous genetic mutation in approximately 80 percent of patients, and in the remaining 20 percent, it is inherited from a parent.
The current treatment landscape includes medications such as growth hormone therapy, surgery, and supportive therapy for achondroplasia. The first drug to be approved for treating achondroplasia is VOXZOGO (Vosoritide) by BioMarin Pharmaceuticals. The European Commission granted marketing authorization to VOXZOGO as a once-daily injection to treat achondroplasia in children from the age of 2 until growth plates are closed, which occurs after puberty when children reach final adult height, in August 2021. In November 2021, US FDA approved VOXZOGO, under accelerated approval pathway, and Rare Pediatric Disease Priority Review Voucher, fastening the approval timeline. Marketing authorization reviews are in process in Japan, Brazil, and Australia, with potential approvals in these countries in 2022.
Achondroplasia Market Key Facts
The diagnosed prevalence of achondroplasia has been increasing in the US due to improved understanding among healthcare professionals, advances in diagnosis, besides an increasing population.
Advances in genetic testing, such as chorionic villus sampling, new-generation sequencing, and amniocentesis, allow for the prenatal diagnosis of achondroplasia.
Management of achondroplasia is symptomatic and requires a multidisciplinary approach involving pharmacological and nonpharmacological interventions. The current treatment landscape has only one approved therapy in the US and Europe, VOXZOGO (vosoritide), while in Japan, growth hormone has also been authorized for the management of achondroplasia; however, its long-term benefits are controversial.
Other therapies like statins, anti-infectives, antihistamines, CNP analogs, etc, are also used to manage symptoms and aid surgical procedures of achondroplasia. Further, surgical therapies like adenotonsillectomy, suboccipital decompression, tracheostomy, laminectomy, and physical therapy play a vital role in managing several complications of achondroplasia.
One of the major concerns in understanding the achondroplasia market is the paucity of evidence to validate interventions used in the daily management of achondroplasia.
There is a lack of curative therapy; however, the presence of clinical and diagnostic guidelines, recommendations, and consensus statements have enabled informed treatment and management, the American Academy of Pediatric’s Health Supervision for Children with Achondroplasia, International Consensus Statement on the Diagnosis, Multidisciplinary Management, and Lifelong Care of Individuals with Achondroplasia, and Japanese Society for Pediatric Endocrinology’s Clinical Practice Guidelines for Achondroplasia are few among them. However, the detailed basis for recommendations and the existing uncertainties are yet to be explored.
Achondroplasia is associated with poor quality of life, substantial economic burdens, negative stereotypes, and social challenges.
In 2022, the US had the largest market size of achondroplasia among the 7MM, accounting for approximately USD 111.85 million, expected to increase further by 2032.
The approved drug is VOXZOGO (vosoritide), a C-type natriuretic peptide (CNP) analog, which acts as a positive regulator of the signaling pathway downstream of FGFR3 to promote endochondral bone growth. The drug is indicated to increase linear growth in children with achondroplasia having open epiphyses.
Although much has been learned about the disorder, limited pharmacological therapies are available, and surgeries have the risk of morbidity. There is an urgent need for an effective therapy that can restore skeletal growth and improve patients’ quality of life.
Ongoing research into achondroplasia has led to the discovery of potential therapies that might alleviate some of the symptoms and complications associated with the condition. Emerging therapies like Ascendis Pharma’s TransCon CNP (navepegritide) and QED Therapeutics (BridgeBio)/Novartis’ infigratinib (BBP-831/BGJ398) have the potential to quench the thirst for effective therapies. The achondroplasia market will change during the forecast period due to the expected approval of these emerging therapies, leading to the entry of new players in the treatment landscape.
TransCon CNP (navepegritide) is a prodrug of CNP administrated subcutaneously that regulates bone growth by maintaining CNP levels into growth plates. The delivery of TransCon CNP requires fewer doses and less quantity of CNP to elicit the therapeutic effects compared to the approved therapy VOXZOGO (vosoritide). Per DelveInsight’s analysts, the drug is anticipated to enter the US market by 2025.
Infigratinib (BBP-831/BGJ398) is an FGFR1-3 tyrosine kinase inhibitor that targets both the pathways, MAPK and STAT1, responsible for the clinical phenotype associated with achondroplasia. Further, its oral and painless administration spares children from the uncomfortable injection approach. The drug is anticipated to enter the US market by 2026 having a medium uptake.
Achondroplasia Market
The market outlook section of the report helps to build a detailed comprehension of the historical, current and forecasted Achondroplasia market size by analyzing the impact of current and emerging pipeline therapies. It also thoroughly assesses the market drivers & barriers, unmet needs, and emerging technologies set to impact the market dynamics.
The report gives complete detail of the Achondroplasia market trend for each marketed drug and mid & late-stage pipeline therapies by evaluating their impact based on the annual cost of therapy, their Mechanism of Action (MOA), Route of Administration (ROA), molecule types, competition with other therapies, brand value, and their impact on the market.
Achondroplasia Epidemiology Assessment
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2019 to 2032. It helps to recognize the causes of current and forecasted epidemiology trends by exploring numerous studies and research. The epidemiology section also provides a detailed analysis of diagnosed and prevalent patient pool, future trends, and views of key opinion leaders.
The Report Covers the Achondroplasia Epidemiology, Segmented as –
Total Prevalent Cases of Achondroplasia [2019–2032]
Age-Specific Cases of Achondroplasia [2019–2032]
Achondroplasia Drugs Uptake and Pipeline Development Activities
The drug uptake section focuses on the uptake rate of potential drugs recently launched in the Achondroplasia market or expected to be launched during the study period. The analysis covers the Achondroplasia market uptake by drugs, patient uptake by therapies, and sales of each drug. Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Achondroplasia pipeline development activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyses recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Learn How the Achondroplasia Market Will Evolve and Grow by 2032 @ https://www.delveinsight.com/report-store/achondroplasia-market
Achondroplasia Therapeutics Analysis
Several major pharma and biotech giants are developing therapies for Achondroplasia. Currently, BioMarin Pharmaceutical is leading the therapeutics market with its Achondroplasia drug candidates in the most advanced stage of clinical development.
Leading Companies in the Achondroplasia Therapeutics Market Include:
Ascendis Pharma
BioMarin Pharmaceutical
Pfizer
PhaseBio Pharmaceuticals
ProLynx Inc
QED Therapeutics (BridgeBio)
Ribomic
Sanofi
And Many Others
Achondroplasia Emerging and Marketed Drugs Covered in the Report Include:
BMN-111: BioMarin Pharmaceutical
CNP-ELP: PhaseBio
Infigratinib: QED Therapeutics (BridgeBio)
PLX138: ProLynx Inc
Recifercept (TA 46): Pfizer
SAR442501: Sanofi
TransCon CNP: Ascendis Pharma
And Many More
The Report Covers the In-depth Assessment of the Emerging Drugs & Key Companies. Download the Sample Report to Learn More @ https://www.delveinsight.com/sample-request/achondroplasia-market
Table of Contents
1. Key Insights
2. Executive Summary
3. Achondroplasia Competitive Intelligence Analysis
4. Achondroplasia Market Overview at a Glance
5. Achondroplasia Disease Background and Overview
6. Achondroplasia Patient Journey
7. Achondroplasia Epidemiology and Patient Population (In the US, EU5, and Japan)
8. Achondroplasia Treatment Algorithm, Current Treatment, and Medical Practices
9. Achondroplasia Unmet Needs
10. Key Endpoints of Achondroplasia Treatment
11. Achondroplasia Marketed Products
12. Achondroplasia Emerging Drugs and Latest Therapeutic Advances
13. Achondroplasia Seven Major Market Analysis
14. Attribute Analysis
15. Achondroplasia Market Outlook (In US, EU5, and Japan)
16. Achondroplasia Access and Reimbursement Overview
17. KOL Views on the Achondroplasia Market
18. Achondroplasia Market Drivers
19. Achondroplasia Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
Download a free sample report @ https://www.delveinsight.com/sample-request/achondroplasia-market
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email: Send Email
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/