“Hemophilia A Market” report has been added to DelveInsight
Hemophilia A Disease Overview
Hemophilia A is a genetic bleeding disorder in which an individual lacks or has low levels of proteins named clotting factor VIII.
Get Free Sample Copy Now- https://www.delveinsight.com/sample-request/hemophilia-a2030-market
Hemophilia A Regions Covered
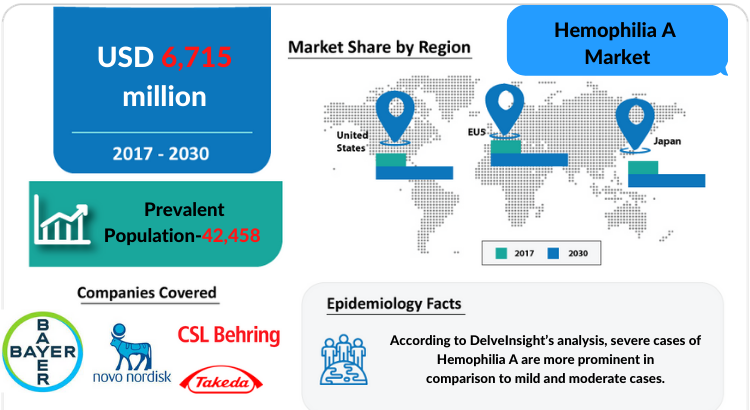
- The United States
- EU5 (Germany, France, Italy, Spain, and the United Kingdom)
- Japan
- Novo Nordisk
- Bayer
- Octapharma
- Takeda
- Sanofi/Sobi
- CSL Behring
- Bayer
- Octapharma
- Pfizer
- Genentech
- HEMA Biologics/LFB Pharmaceuticals
- And many others
Hemophilia A Drugs
- Esperoct (N8-GP; Turoctocog alfa pegol)
- Jivi (formerly BAY94-9027)
- Wilate
- Adynovate (Adynovi; BAX 855)
- Eloctate [Antihemophilic Factor (Recombinant), Fc Fusion Protein; Elocta (efmoroctocog alfa)]
- Afstyla (Lonoctocog alfa)
- Kovaltry (BAY 81-8973)
- Nuwiq (simoctocog alfa)
Hemophilia A Treatment Market
The mainstay treatment option has long been FVIII replacement therapy. Initially, FVIII replacement was accomplished by donated whole blood, subsequently by plasma and currently by recombinant human FVIII (rFVIII) replacement therapies, which revolutionized the treatment of Hemophilia A.
About 30% of severe hemophilia A patients develop neutralizing anti-FVIII alloantibodies (inhibitors), which render the FVIII replacement ineffective. The standard of care therapy for patients with inhibitors is to induce immune tolerance with high-dose, high-frequency FVIII and treatment with bypassing agents (e.g. recombinant activated factor VII such as NovoSeven, FEIBA).
Hemophilia A Market Research
As per DelveInsight estimation, the future of hemophilia treatment is continuing to trend toward extended half-life therapies as well as more novel approaches including siRNA, bi-specific antibodies and gene therapy. If these therapies are ultimately successfully commercialized they have the potential to transform the current standard of care for hemophilia A patients
Hemophilia A Market Industry
Hemophilia A is a life-long condition. Currently, there is no cure, but researchers are actively engaged in finding the cure through gene therapy. One hope is that by inserting a healthy version of the defective blood factor gene, a person with hemophilia will be able to produce reasonable amounts of a factor on their own.
Hemophilia A Market Trends
Generally, Hemophilia A patients are provided with “On Demand” and “Prophylaxis” treatment. But Prophylaxis treatment option has gained importance in comparison to the on-demand treatment options. Currently, the major treatment options of Hemophilia A are:
Currently, the major treatment options of Hemophilia A are Factor Replacement Concentrates, the source of which is either recombinant DNA technology or Human plasma-derive, and Bypassing agents. Moreover, this is an off-label treatment option, wherein Desmopressin Acetate (DDAVP) and Adjunctive therapies are also available for the management of Hemophilia A.
Hemophilia A Market Highlights
- In the coming years, Hemophilia A market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence Hemophilia A R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing therapies for Hemophilia A. Launch of emerging therapies will significantly impact the Hemophilia A market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Hemophilia A
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Get Free Sample Copy Now- https://www.delveinsight.com/sample-request/hemophilia-a2030-market
Table of content
1. Key Insights
2. Executive Summary of Hemophilia A
3. Competitive Intelligence Analysis for Hemophilia A
4. Hemophilia A: Market Overview at a Glance
5. Hemophilia A: Disease Background and Overview
6. Patient Journey
7. Hemophilia A Epidemiology and Patient Population
8. Treatment Algorithm, Current Treatment, and Medical Practices
9. Unmet Needs
10. Key Endpoints of Hemophilia A Treatment
11. Marketed Products
12. Emerging Therapies
13. Hemophilia A: Seven Major Market Analysis
14. Attribute analysis
15. 7MM: Market Outlook
16. Access and Reimbursement Overview of Hemophilia A
17. KOL Views
18. Market Drivers
19. Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
23. About DelveInsight
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Shruti Thakur
Email: Send Email
Phone: 9193216187
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: www.delveinsight.com/