(Albany, USA) DelveInsight’s “Homozygous Familial Hypercholesterolemia Market Insights, Epidemiology, and Market Forecast-2034″ report offers an in-depth understanding of the Homozygous Familial Hypercholesterolemia, historical and forecasted epidemiology as well as the Homozygous Familial Hypercholesterolemia market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Homozygous Familial Hypercholesterolemia market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Homozygous Familial Hypercholesterolemia Market Forecast
Some of the key facts of the Homozygous Familial Hypercholesterolemia Market Report:
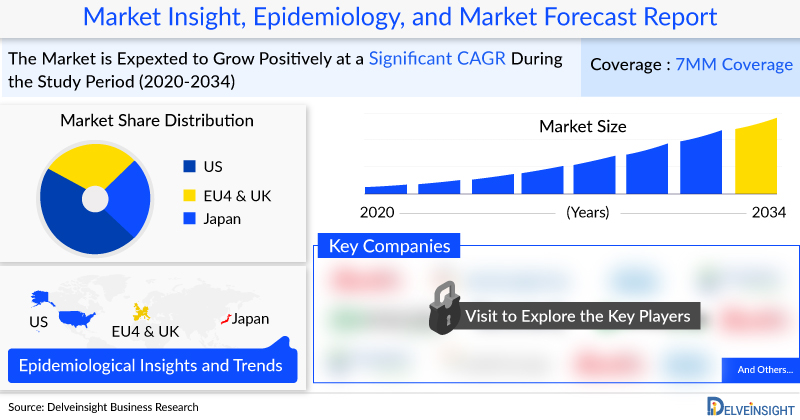
- The Homozygous Familial Hypercholesterolemia market size was valued approximately USD 108 million in 2022 and is anticipated to grow with a significant CAGR during the study period (2020-2034)
- DelveInsight’s 2022 analysis indicates that there were approximately 2,845 diagnosed prevalent cases of HoFH in the 7MM. This number is projected to rise significantly by 2034, with a notable CAGR throughout the study period (2020–2034).
- Estimates from DelveInsight’s epidemiology model suggest that there were around 1,349 diagnosed prevalent cases of HoFH in the US in 2022. This number is projected to increase by 2034 over the study period (2020–2034).
- In the 7MM, the EU4 and the UK accounted for about 38% of the total diagnosed prevalent cases of HoFH in 2022, with expectations for this figure to rise further during the study period.
- According to DelveInsight’s analysis, the United States alone represented approximately 47% of all diagnosed prevalent cases of HoFH among the 7MM in 2022.
- Key Homozygous Familial Hypercholesterolemia Companies: Arrowhead Pharmaceutical, Novartis, Alnylam Pharmaceutical, LIB Therapeutics, Amryt Pharma, Novartis, Ultragenyx Pharmaceutical, Arrowhead Pharmaceuticals, Sanofi, Cerenis Therapeutics, Aegerion Pharmaceuticals, The Medicines Company, Akeso, Aegerion Pharmaceuticals, and others
- Key Homozygous Familial Hypercholesterolemia Therapies: ARO-ANG3, LEQVIO (inclisiran/KJX839), Lerodalcibep (LIB003), Lomitapide, Inclisiran, Evinacumab, ARO-ANG 3 Injection, Alirocumab, CER-001, lomitapide, ALN-PCSSC, AK102, AEGR-733, and others
- The Homozygous Familial Hypercholesterolemia epidemiology based on mutation-specific cases analyzed that a mutation in the LDLR gene accounted for the highest diagnosed prevalent cases in the US
- The Homozygous Familial Hypercholesterolemia market is expected to surge due to the disease’s increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Homozygous Familial Hypercholesterolemia pipeline products will significantly revolutionize the Homozygous Familial Hypercholesterolemia market dynamics.
- On March 22, 2023, Regeneron Pharmaceuticals, Inc. announced that the FDA has extended the approval of Evkeeza (evinacumab-dgnb) as an addition to other lipid-lowering medicines in children aged 5 to 11 with homozygous familial hypercholesterolemia (HoFH). Evkeeza is the first angiopoietin-like 3 (ANGPTL3) inhibitor medication approved for children as young as 5 years old to decrease dangerously high levels of low-density lipoprotein cholesterol (LDL-C) caused by HoFH.
Homozygous Familial Hypercholesterolemia Overview
Homozygous familial hypercholesterolemia (HoFH) is a rare genetic disorder characterized by extremely high levels of low-density lipoprotein cholesterol (LDL-C) from birth, leading to an increased risk of early-onset cardiovascular disease. It occurs in individuals who inherit defective copies of the LDL receptor (LDLR) gene or related genes from both parents, impairing the body’s ability to remove LDL-C from the bloodstream.
Patients with HoFH often present with LDL-C levels exceeding 500 mg/dL and may develop symptoms of atherosclerosis in childhood or adolescence. Physical signs can include xanthomas (cholesterol deposits in tendons and skin) and corneal arcus (cholesterol deposits around the cornea). Severe complications, such as premature heart attacks, angina, or aortic valve disease, can occur early in life.
Diagnosis is based on clinical presentation, family history, and genetic testing to confirm mutations. Early detection is crucial for effective management.
Treatment involves aggressive lipid-lowering strategies, including high-dose statins, ezetimibe, PCSK9 inhibitors, and novel therapies such as evinacumab or lomitapide. Lipoprotein apheresis, a procedure to physically remove LDL-C from the blood, is often necessary. Early and intensive management can improve outcomes and reduce the risk of life-threatening cardiovascular events in HoFH patients.
Get a Free sample for the Homozygous Familial Hypercholesterolemia Market Forecast, Size & Share Analysis Report: https://www.delveinsight.com/sample-request/homozygous-familial-hypercholesterolemia-market
Homozygous Familial Hypercholesterolemia Epidemiology
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2020 to 2034. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. The epidemiology section also provides a detailed analysis of the diagnosed patient pool and future trends.
Homozygous Familial Hypercholesterolemia Epidemiology Segmentation:
The Homozygous Familial Hypercholesterolemia market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Diagnosed Prevalent Cases of HoFH in the 7MM
- Mutation-specific Diagnosed Prevalent Cases of HoFHin the 7MM
Download the report to understand which factors are driving Homozygous Familial Hypercholesterolemia epidemiology trends @ Homozygous Familial Hypercholesterolemia Epidemiology Forecast
Homozygous Familial Hypercholesterolemia Drugs Uptake and Pipeline Development Activities
The drugs uptake section focuses on the rate of uptake of the potential drugs recently launched in the Homozygous Familial Hypercholesterolemia market or expected to get launched during the study period. The analysis covers Homozygous Familial Hypercholesterolemia market uptake by drugs, patient uptake by therapies, and sales of each drug.
Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Homozygous Familial Hypercholesterolemia Pipeline Development Activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyzes recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Homozygous Familial Hypercholesterolemia Therapies and Key Companies
- ARO-ANG3: Arrowhead Pharmaceutical
- LEQVIO (inclisiran/KJX839: Novartis/ Alnylam Pharmaceutical
- Lerodalcibep (LIB003): LIB Therapeutics
- Lomitapide: Amryt Pharma
- Inclisiran: Novartis
- Evinacumab: Ultragenyx Pharmaceutical
- ARO-ANG 3 Injection: Arrowhead Pharmaceuticals
- Alirocumab: Sanofi
- CER-001: Cerenis Therapeutics
- lomitapide: Aegerion Pharmaceuticals
- ALN-PCSSC: The Medicines Company
- AK102: Akeso
- AEGR-733: Aegerion Pharmaceuticals
Request for sample report @ Homozygous Familial Hypercholesterolemia Treatment Market
Homozygous Familial Hypercholesterolemia Market Strengths
- The clarity in disease understanding and pathogenesis has driven the development of novel pharmacological options like PCSK9 inhibitors, ANGPTL3 inhibitors, and MTP inhibitor
- Updated international diagnostic and clinical guidelines for FH and HoFH enable evidence-based therapeutic approaches and screening strategies for early identification
- Advances in research have led to the discovery of novel molecules like siRNA and recombinant fusion protein that may offer novel options to lower LDL significantly
Homozygous Familial Hypercholesterolemia Market Opportunities
- Preclinical studies have yielded gene therapy and CRISPR-based gene editing approaches; conducting further research and clinical trials may offer curative therapy.
- Current therapies do not impart sufficient cholesterol-lowering allowing pharma players to bring newer, more potent, and better LDL-C lowering therapies and prevent ASCVD in HoFH patients.
- Advancements in healthcare technologies and digital health solutions can potentially enhance early detection and personalized management of HoFH.
Scope of the Homozygous Familial Hypercholesterolemia Market Report
- Study Period: 2020–2034
- Coverage: 7MM [The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan]
- Key Homozygous Familial Hypercholesterolemia Companies: Arrowhead Pharmaceutical, Novartis, Alnylam Pharmaceutical, LIB Therapeutics, Amryt Pharma, Novartis, Ultragenyx Pharmaceutical, Arrowhead Pharmaceuticals, Sanofi, Cerenis Therapeutics, Aegerion Pharmaceuticals, The Medicines Company, Akeso, Aegerion Pharmaceuticals, and others
- Key Homozygous Familial Hypercholesterolemia Therapies: ARO-ANG3, LEQVIO (inclisiran/KJX839), Lerodalcibep (LIB003), Lomitapide, Inclisiran, Evinacumab, ARO-ANG 3 Injection, Alirocumab, CER-001, lomitapide, ALN-PCSSC, AK102, AEGR-733, and others
- Homozygous Familial Hypercholesterolemia Therapeutic Assessment: Homozygous Familial Hypercholesterolemia current marketed and Homozygous Familial Hypercholesterolemia emerging therapies
- Homozygous Familial Hypercholesterolemia Market Dynamics: Homozygous Familial Hypercholesterolemia market drivers and Homozygous Familial Hypercholesterolemia market barriers
- Competitive Intelligence Analysis: SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies
- Homozygous Familial Hypercholesterolemia Unmet Needs, KOL’s views, Analyst’s views, Homozygous Familial Hypercholesterolemia Market Access and Reimbursement
To know more about Homozygous Familial Hypercholesterolemia companies working in the treatment market, visit @ Homozygous Familial Hypercholesterolemia Clinical Trials and Treatments
Table of Contents
1. Homozygous Familial Hypercholesterolemia Market Report Introduction
2. Executive Summary for Homozygous Familial Hypercholesterolemia
3. SWOT analysis of Homozygous Familial Hypercholesterolemia
4. Homozygous Familial Hypercholesterolemia Patient Share (%) Overview at a Glance
5. Homozygous Familial Hypercholesterolemia Market Overview at a Glance
6. Homozygous Familial Hypercholesterolemia Disease Background and Overview
7. Homozygous Familial Hypercholesterolemia Epidemiology and Patient Population
8. Country-Specific Patient Population of Homozygous Familial Hypercholesterolemia
9. Homozygous Familial Hypercholesterolemia Current Treatment and Medical Practices
10. Homozygous Familial Hypercholesterolemia Unmet Needs
11. Homozygous Familial Hypercholesterolemia Emerging Therapies
12. Homozygous Familial Hypercholesterolemia Market Outlook
13. Country-Wise Homozygous Familial Hypercholesterolemia Market Analysis (2020–2034)
14. Homozygous Familial Hypercholesterolemia Market Access and Reimbursement of Therapies
15. Homozygous Familial Hypercholesterolemia Market Drivers
16. Homozygous Familial Hypercholesterolemia Market Barriers
17. Homozygous Familial Hypercholesterolemia Appendix
18. Homozygous Familial Hypercholesterolemia Report Methodology
19. DelveInsight Capabilities
20. Disclaimer
21. About DelveInsight
About DelveInsight
DelveInsight is a leading Healthcare Business Consultant, and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate the business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email: Send Email
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting/primary-research-services