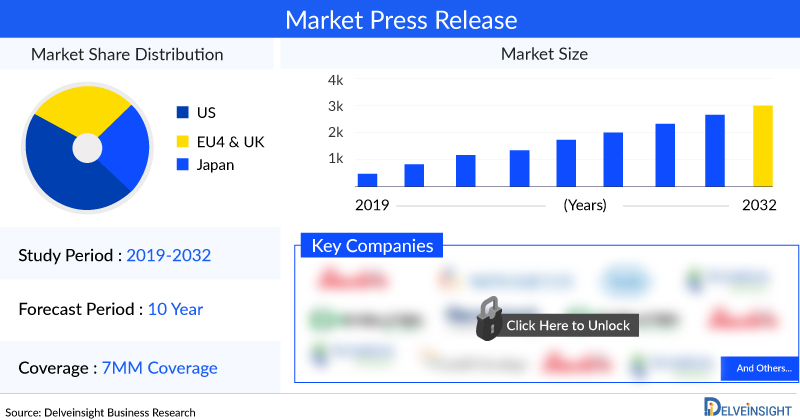
DelveInsight’s “Phenylketonuria Market Insights, Epidemiology, and Market Forecast 2034” report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the Phenylketonuria market size, share, trends, and growth opportunities in the seven major markets (7MM) (i.e., the United States, EU4 (Germany, Spain, Italy, France), the United Kingdom and Japan).
The Phenylketonuria market report covers emerging drugs, current treatment practices, market share of individual therapies, and current & forecasted market size from 2020 to 2034. It also evaluates the current treatment practice/algorithm, key drivers & barriers impacting the market growth, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Key highlights from the Phenylketonuria Market Report:
-
The 7MM accounted for a market size of approximately 700 USD million in 2023.
-
Various key players are leading the treatment landscape of phenylketonuria, such as Asubio-Pharma/BioMarin-Pharmaceutical, PTC Therapeutics, Synlogic, and others. The details of the country-wise and therapy-wise market size have been provided below.
-
In 2023, the United States accounted for the largest market size among the 7MM, making ~60% of the total market size of the 7MM.
-
Key phenylketonuria companies working in the phenylketonuria market are PTC Therapeutics, Homology Medicines, Synlogic, BioMarin Pharmaceuticals, and others.
-
Synlogic’s SYNB1934 is expected to become a key player of the PKU market during the forecast period (2024–2034).
-
Among the EU4 and the UK, Germany had the largest market size with ~USD 70 million in 2023, while Spain had the smallest market size of PKU.
-
Among the 7MM, the United States had the highest number of diagnosed prevalent cases of phenylketonuria (PKU) with 18,000 cases in 2023.
-
The age‐specific data revealed that the highest number of patients affected with PKU was found in the age group of
-
Currently, the market landscape offers only two approved therapies, both of which are owned by BioMarin. The first is KUVAN (sapropterin), a synthetic form of tetrahydrobiopterin (BH4), which acts by increasing phenylalanine hydroxylase (PAH) activity. The second therapy is PALYNZIQ (pegvaliase), a phenylalanine ammonia-lyase (PAL) enzyme that temporarily restores the levels of PAH and reduces blood phenylalanine concentrations.
-
In March 2024, PTC submitted a Marketing Authorization Application (MAA) to the EMA for sepiapterin. Additionally, the company anticipates submitting a NDA to the FDA in the third quarter of 2024 and completing regulatory submissions in Japan within the same year
-
Another drug, Synlogic’s SYNB1934 (Currently in Phase II), is expected to become a key player during the forecast period (2024–2034), owing to its novel MoA, impressive efficacy data, and a patient compliant RoA.
-
The expected launch of potential therapies may increase market size in the coming years, assisted by an increase in the diagnosed prevalent population of PKU.
Read detailed insights on Phenylketonuria market outlook 2034 @ https://www.delveinsight.com/sample-request/phenylketonuria-pku-market
Phenylketonuria Overview
Phenylketonuria (PKU) is a rare genetic disorder characterized by the accumulation of an amino acid called phenylalanine in the body. Amino acids, which are the building blocks of proteins, are found in all proteins and some artificial sweeteners. PKU can present with a range of symptoms, from mild to severe. The most severe form, known as classic PKU, may not show symptoms until several months after birth. A less severe variant, called variant PKU or non-PKU hyperphenylalaninemia, results in elevated levels of phenylalanine but typically causes only mild symptoms.
PKU is usually diagnosed shortly after birth through neonatal screening programs in many developed countries. In regions with expanded newborn screening, PKU is detected by measuring phenylalanine (Phe) and tyrosine (Tyr) levels in dried blood spots using tandem mass spectrometry. The traditional bacterial inhibition assay (Guthrie test) is a manual and semi-quantitative method that has been largely replaced by more modern, automated techniques. Some laboratories now employ fluorimetric tests, which are quantitative, automated, and reliable.
Sapropterin dihydrochloride, a synthetic form of tetrahydrobiopterin (BH4), has been introduced as a supplementary treatment alongside dietary management for PKU. BH4 is a natural cofactor for the enzyme phenylalanine hydroxylase (PAH), which converts phenylalanine into tyrosine. In individuals with PKU, PAH activity is deficient or absent. BH4 supplementation can help activate any residual PAH enzyme activity, enhancing the metabolism of phenylalanine and reducing its levels in some patients.
KUVAN (sapropterin) is an approved treatment for PKU. By providing the BH4 cofactor, KUVAN helps to improve the oxidative metabolism of phenylalanine, thereby lowering phenylalanine levels in certain individuals with the condition.
Phenylketonuria Epidemiology Insights:
-
Most cases of phenylketonuria (PKU) are identified in infants due to widespread newborn screening programs. Data indicate that the majority of PKU patients are aged 14 years or younger, representing nearly 60% of cases across the seven major markets (7MM) in 2023.
-
According to DelveInsight’s estimates, in 2023, missense mutations accounted for 60% of the total diagnosed PKU cases in the 7MM, while nonsense mutations represented 5%.
-
Among the EU4 countries and the UK, Germany reported the highest number of diagnosed PKU cases, followed by France. Spain had the fewest cases in 2023.
In the United States, classic PKU was the most prevalent form, with approximately 11,000 cases recorded in 2023.
Phenylketonuria Marketed Therapies
-
KUVAN (Sapropterin Hydrochloride): Asubio-Pharma/BioMarin-Pharmaceutical
-
PALYNZIQ (pegvaliase-pqpz/rAvPAL-PEG/BMN 165): BioMarin Pharmaceutical
Phenylketonuria Emerging Therapies
-
Sepiapterin (PTC923): PTC Therapeutics
-
SYNB1934: Synlogic
Learn How the Phenylketonuria Market Will Evolve and Grow by 2034 @ https://www.delveinsight.com/sample-request/phenylketonuria-pku-market
Phenylketonuria Market Outlook
The primary objective of PKU treatment is to maintain plasma phenylalanine levels between 120−360 µmol/L (2−6 mg/dL), typically achieved through a meticulously planned and monitored diet.
In 2007, the US FDA approved KUVAN (sapropterin hydrochloride) for the treatment of PKU. KUVAN is an oral formulation of tetrahydrobiopterin (BH4), a natural cofactor for the PAH enzyme, which helps enhance the activity of any residual PAH enzyme, facilitating the conversion of phenylalanine into tyrosine. KUVAN is used alongside a phenylalanine-restricted diet and is produced by BioMarin Pharmaceutical. However, KUVAN is not effective for all PKU patients and shows the best results in children with mild forms of the disorder.
Research is ongoing to identify additional treatment options for PKU. These include large neutral amino acid supplementation, which may reduce the brain’s uptake of phenylalanine, and enzyme replacement therapy, which aims to introduce a substance that mimics the action of the enzyme responsible for breaking down phenylalanine.
Current studies indicate that PKU treatment involves a combination of approaches. The development pipeline includes several potential monotherapies. The PKU therapeutics market is anticipated to expand significantly during the forecast period (2024–2034).
Leading Players in the Phenylketonuria Therapeutics Market Include:
Phenylketonuria Companies working in the market are PTC Therapeutics, Homology Medicines, Synlogic, BioMarin Pharmaceuticals, and others.
Phenylketonuria Report Covers the In-depth Assessment of the Emerging Phenylketonuria Drugs & Key Companies. Download the Phenylketonuria Market Sample Report to Learn More @ https://www.delveinsight.com/report-store/phenylketonuria-pku-market
Table of Contents
1. Key Insights
2. Executive Summary
3. Phenylketonuria Competitive Intelligence Analysis
4. Phenylketonuria Market Overview at a Glance
5. Phenylketonuria Disease Background and Overview
6. Phenylketonuria Patient Journey
7. Phenylketonuria Epidemiology and Patient Population (In the US, EU5, and Japan)
8. Phenylketonuria Treatment Algorithm, Current Treatment, and Medical Practices
9. Phenylketonuria Unmet Needs
10. Key Endpoints of Phenylketonuria Treatment
11. Phenylketonuria Marketed Products
12. Phenylketonuria Emerging Drugs and Latest Therapeutic Advances
13. Phenylketonuria Seven Major Market Analysis
14. Attribute Analysis
15. Phenylketonuria Market Outlook (In US, EU5, and Japan)
16. Phenylketonuria Access and Reimbursement Overview
17. KOL Views on the Phenylketonuria Market
18. Phenylketonuria Market Drivers
19. Phenylketonuria Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
Trending Reports by DelveInsight:
Adalimumab Biosimilar Market | Arbovirus Infection Market | Artificial Pancreas Device System Market | Dental Equipment Market | Gluten Sensitivity Market | Hypothyroidism Market | Inflammatory Bowel Disease Market | Mayus Kinase Jak Inhibitors Market | Mild Dry Eye Market | Mucopolysaccharidosis Market | Oncolytic Virus Cancer Therapy Market | Pyoderma Gangrenosum Market | Transdermal Drug Delivery Devices Market
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email: Send Email
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/