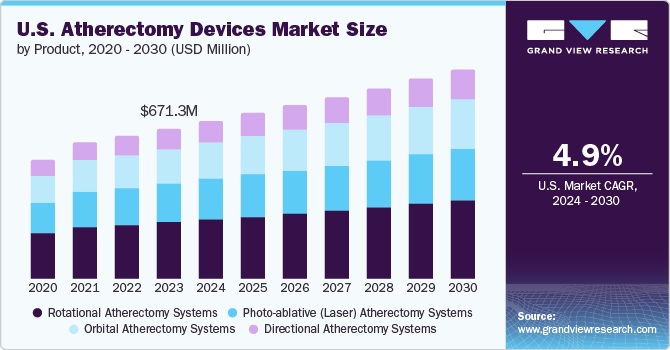
According to a report “U.S. Atherectomy Devices Market Size, Share & Trends Analysis Report By Type (Laser, Directional, Rotational, Orbital), By End Use (Office-based Labs, Out-patient Facility, In-patient Facility), And Segment Forecasts, 2019 – 2025” published by Grand View Research, Inc, The U.S. Atherectomy Devices Market size is expected to reach USD 626.1 million by 2025 expanding at a CAGR of 6.6%, according to a new report by Grand View Research, Inc. The regional market is expected to witness lucrative growth due to the rising prevalence of target diseases and preference for endovascular procedures. Moreover, rising demand for minimally invasive procedure will boost the market growth. Such procedures are less painful and allow quicker recovery than invasive procedures.
Key Takeaways from the report:
- Adoption of atherectomy increased sharply in the past due to increased reimbursement for the procedure. According to Elsevier Inc., there was a rise in atherectomy volume for Medicare beneficiaries to 76% in 2011–2015
- Directional atherectomy devices accounted for the largest share of the market in 2017 as the device exhibited 95% successful removal of lesions and 94% of patients did not exhibit adverse effects
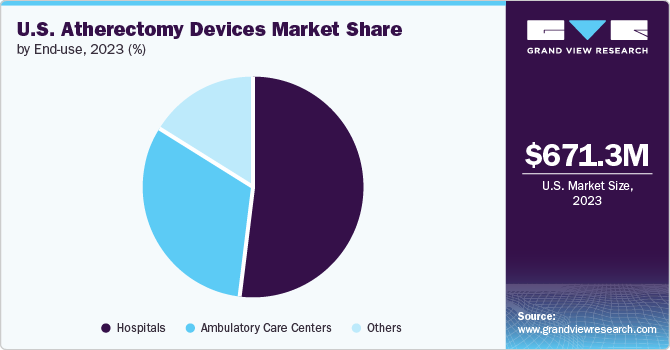
- Office-Based Labs (OBLs) is anticipated to be the fastest-growing segment in the forecast period due to a greater number of Peripheral Vascular Interventional (PVI) procedures being performed in this setting
- In addition, lesser number of complications and follow-up procedures lowers the cost and increases the adoption of new technologies delivering better health outcomes, which is anticipated to propel the market growth in the coming years
- Several ongoing trials and their positive results facilitate penetration of novel technologies. For instance, in March 2017, Cardiovascular Systems, Inc. enrolled for its latest clinical trial, ECLIPSE, which was perceived to be the largest trial conducted to study coronary atherectomy
- Some of the key companies are Spectranetics Corp.; Boston Scientific Corp.; Cardinal Health, Inc.; Straub Medical AG; Terumo Medical Corp.; Cardiovascular Systems, Inc.; Medtronic PLC; Avinger, Inc.; ST. JUDE MEDICAL, Inc.
Browse More Reports in Medical Device Industry:
Insulin Delivery Devices Market – One of the foremost factors contributing to the growth of the Insulin Delivery Devices Market is the surging number of diabetics due to aging, obesity, and unhealthy lifestyles.
Coagulation Analyzer Market – Increasing geriatric population base, rising prevalence of chronic diseases and rapid growth in foreign investments are expected to be witnessed Coagulation Analyzer Market over the forecast period
Other advantages, such as higher patient satisfaction owing to fewer incision wounds, few post-surgical complications, and low mortality rates, are also driving the atherectomy devices market growth. Technological advancements allowing early diagnosis are also likely to augment the market growth in the near future. For instance, in January 2017, Medtronic received CE mark approval for HawkOne directional atherectomy system for treating patients suffering from Peripheral Artery Disease (PAD).
Grand View Research has segmented the U.S. atherectomy devices market on the basis of type and end use:
Atherectomy Devices Market Type (Revenue, USD Million, 2014 – 2025)
- Directional Atherectomy
- Rotational Atherectomy
- Orbital Atherectomy
- Laser Atherectomy
Atherectomy Devices Market End Use (Revenue, USD Million, 2014 – 2025)
- In-patient Facility
- Office-based Labs (OBLs)
- Out-patient Facility
Explore the BI enabled intuitive market research database, Navigate with Grand View Compass, by Grand View Research, Inc.
About Grand View Research
Grand View Research provides syndicated as well as customized research reports and consulting services on 46 industries across 25 major countries worldwide. This U.S.-based market research and consulting company is registered in California and headquartered in San Francisco. Comprising over 425 analysts and consultants, the company adds 1200+ market research reports to its extensive database each year. Supported by an interactive market intelligence platform, the team at Grand View Research guides Fortune 500 companies and prominent academic institutes in comprehending the global and regional business environment and carefully identifying future opportunities.
For more information: www.grandviewresearch.com
Media Contact
Company Name: Grand View Research, Inc.
Contact Person: Sherry James, Corporate Sales Specialist – U.S.A.
Email: Send Email
Phone: 1-415-349-0058, Toll Free: 1-888-202-9519
Address:201, Spear Street, 1100
City: San Francisco
State: California
Country: United States
Website: www.grandviewresearch.com/industry-analysis/us-atherectomy-devices-market

